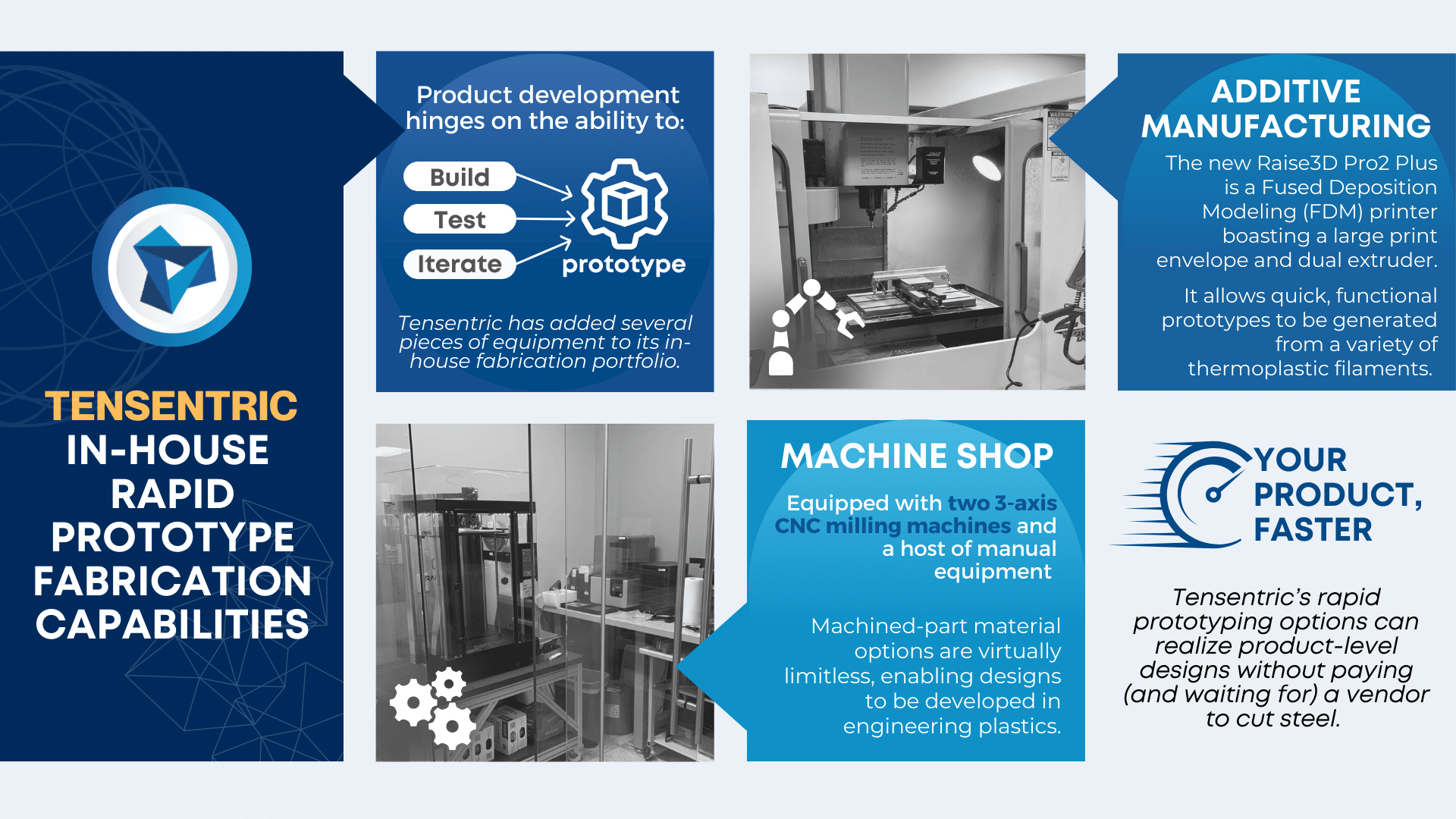
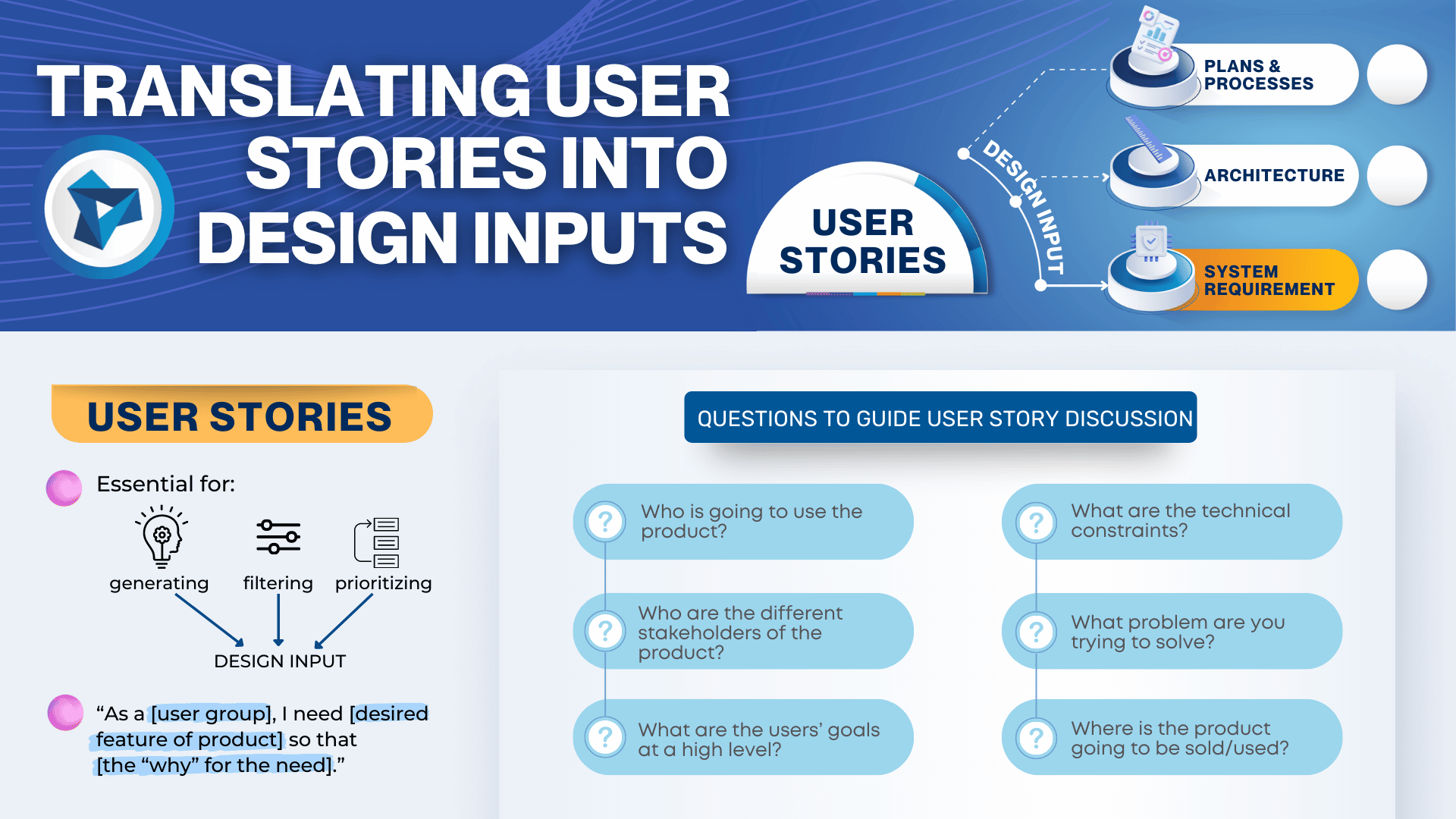
Thinking
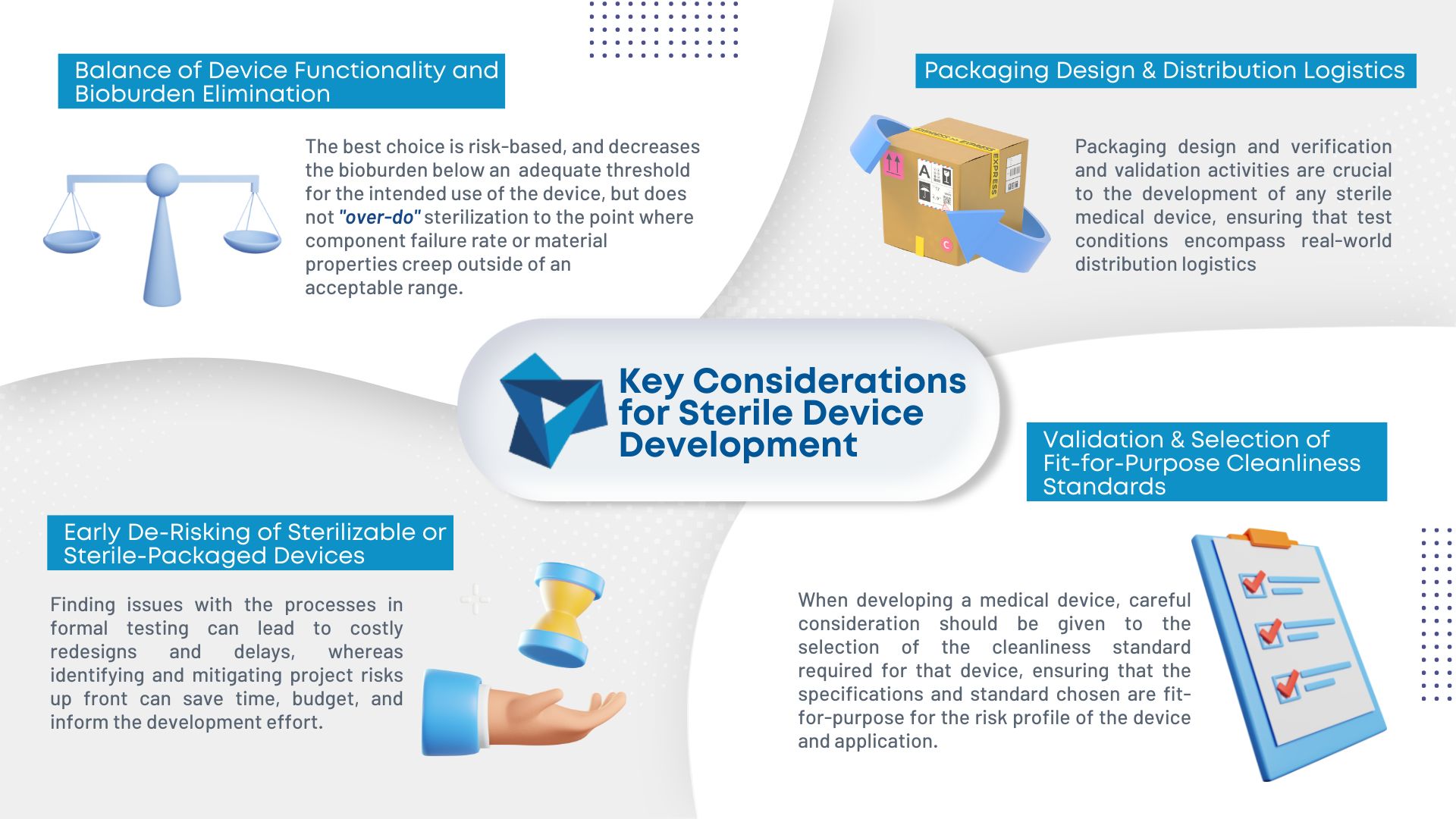
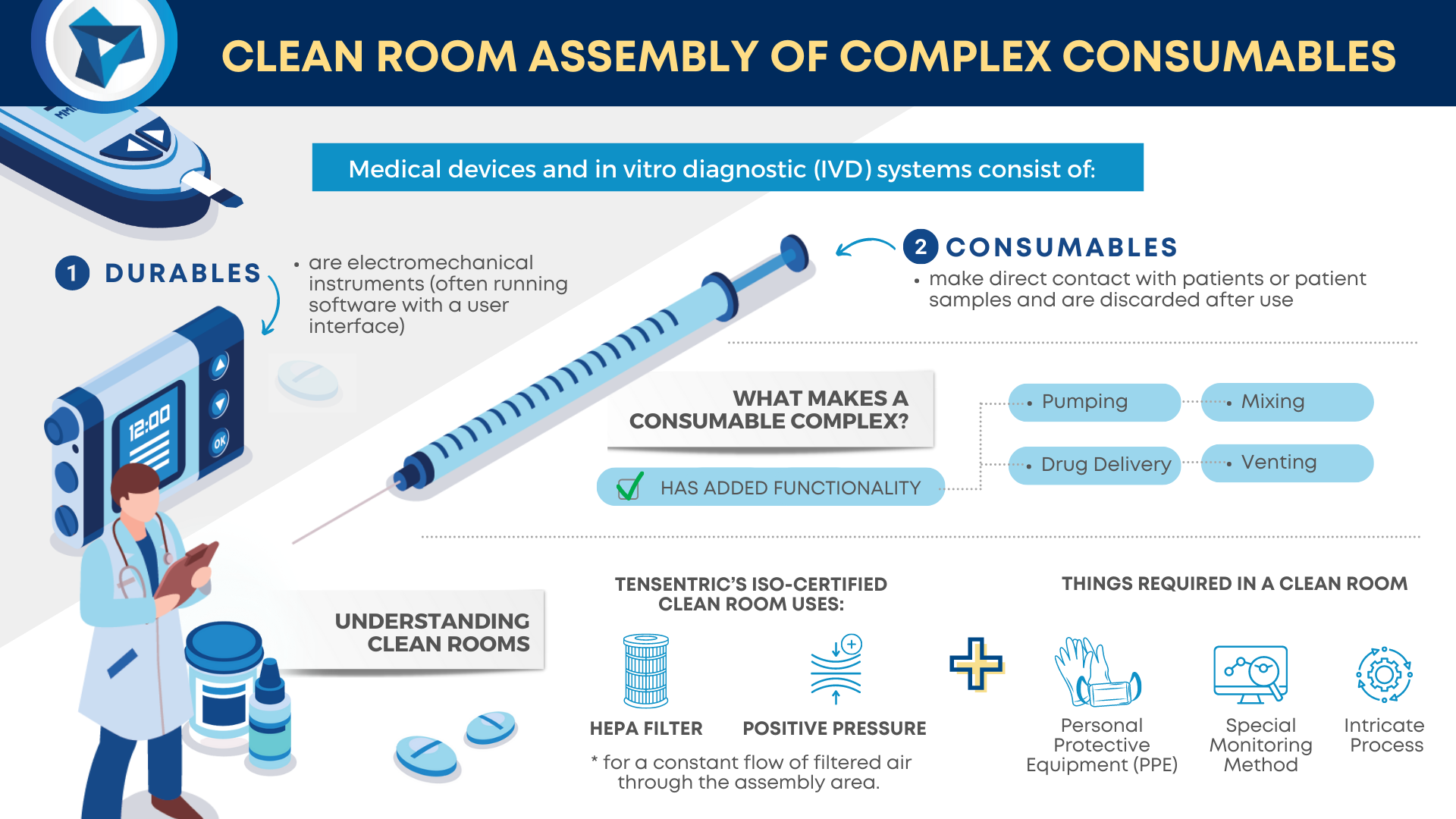
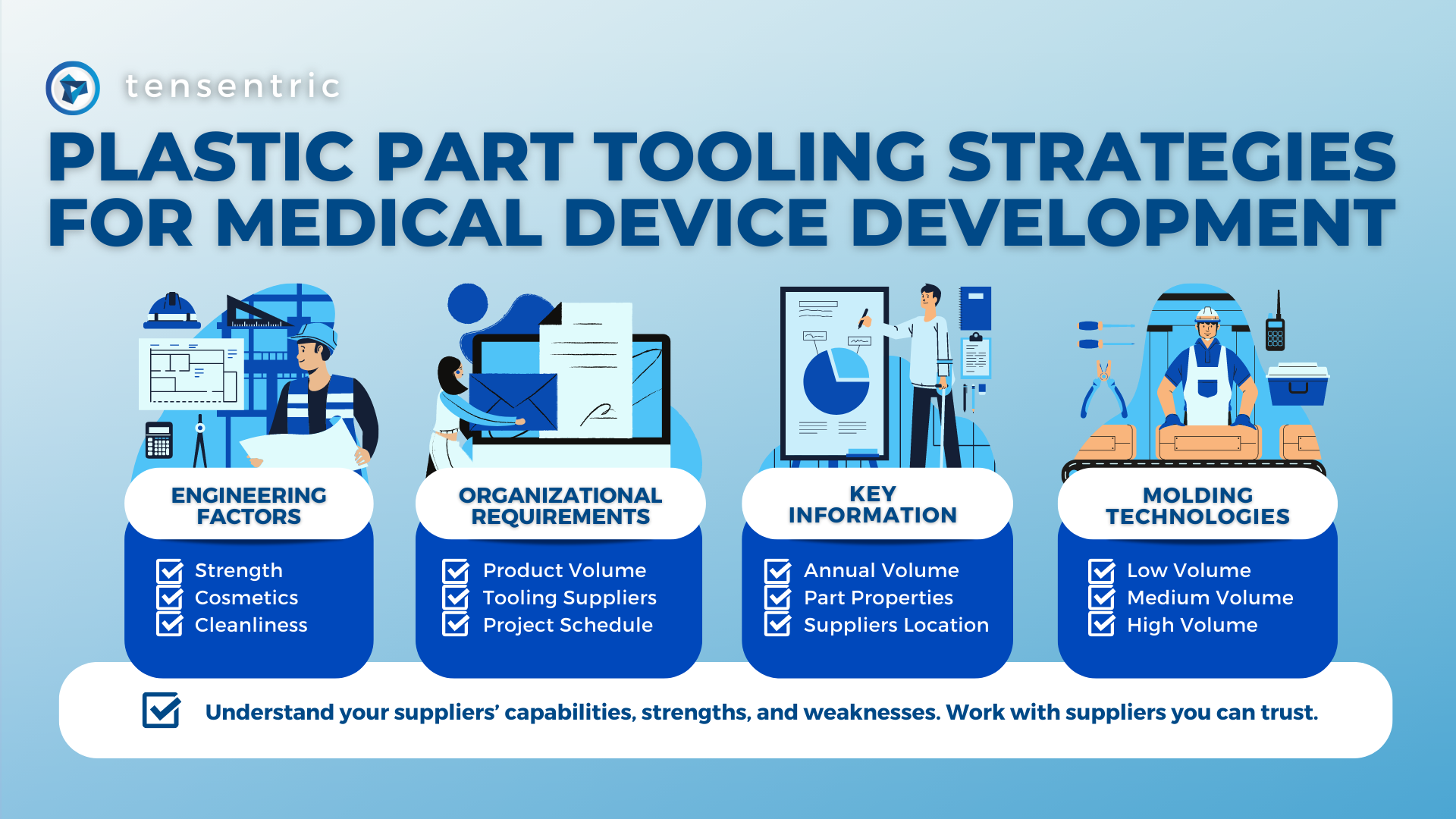
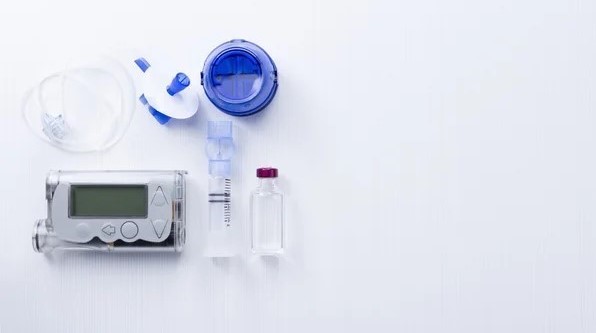
As medical device consumables become more complex, so does manufacturing them. Sterilization is becoming an increasing concern in the industry. To ensure the proper sterilization is in place, there are four key considerations during development: Balance of device functionality and bioburden elimination: Different sterilization methods have different degradation effects on materials making it important to […]
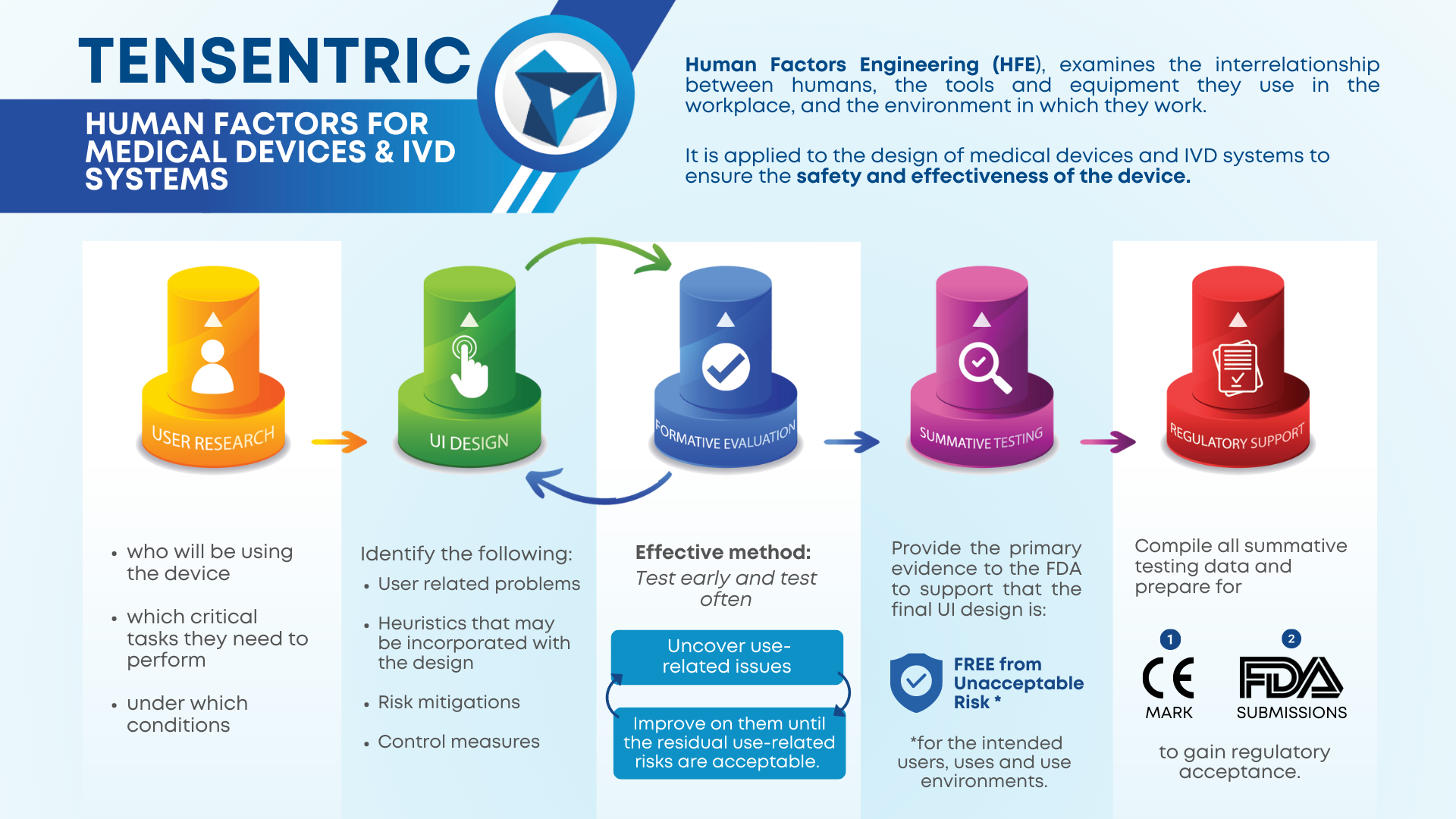
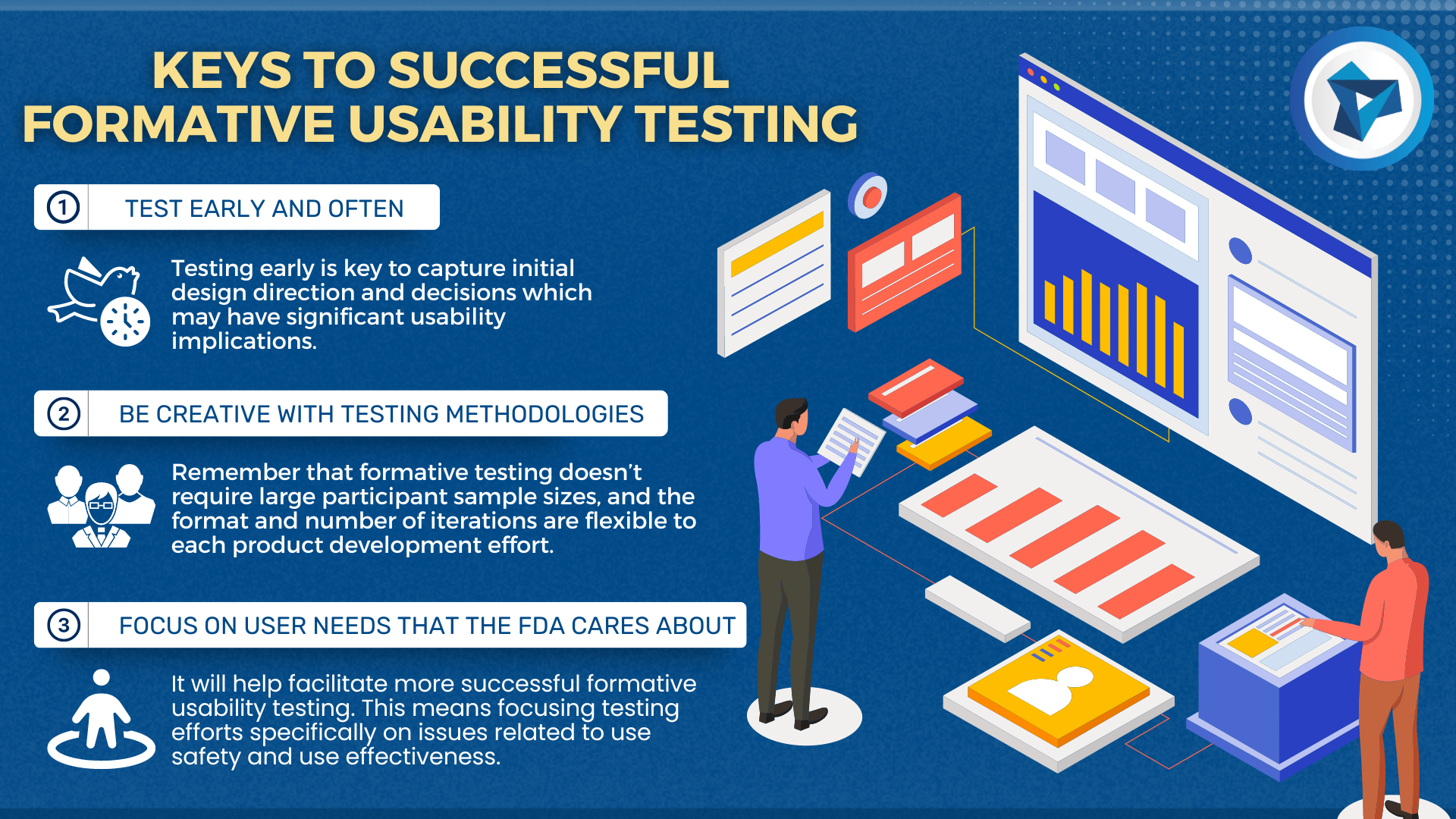
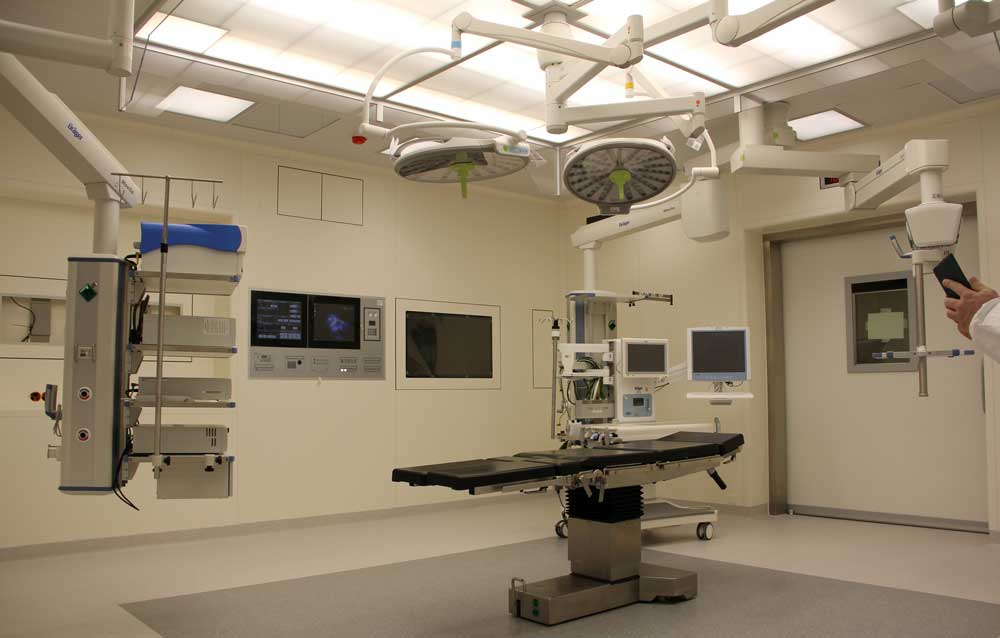
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of life. Therefore, it is essential that medical devices and IVD systems be designed as safe and effective for the intended users, uses and […]
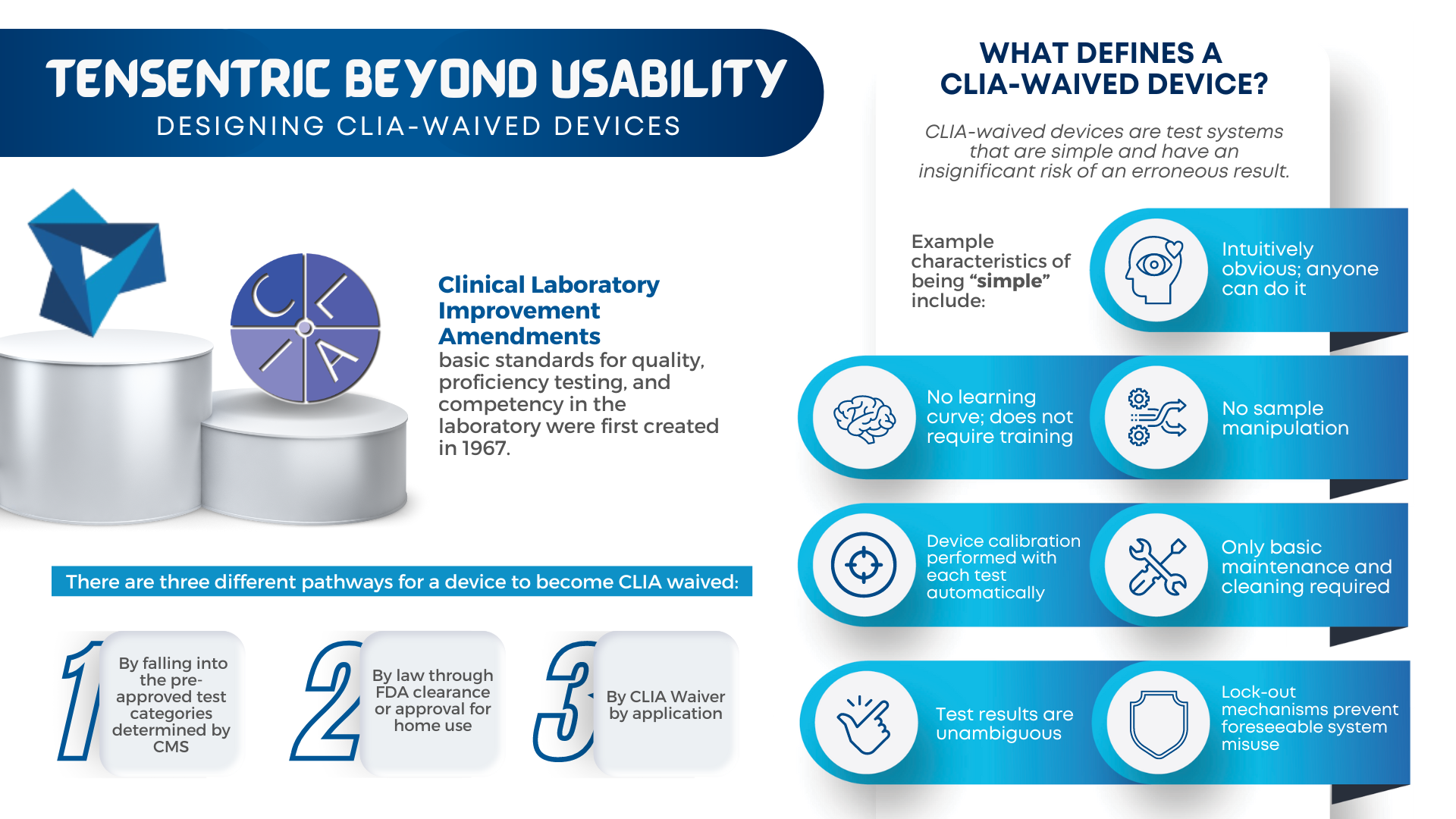
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important for better and faster patient care. For manufacturers, it vastly broadens the potential market to include non-lab and non-hospital clinics, urgent care centers, medical facilities […]
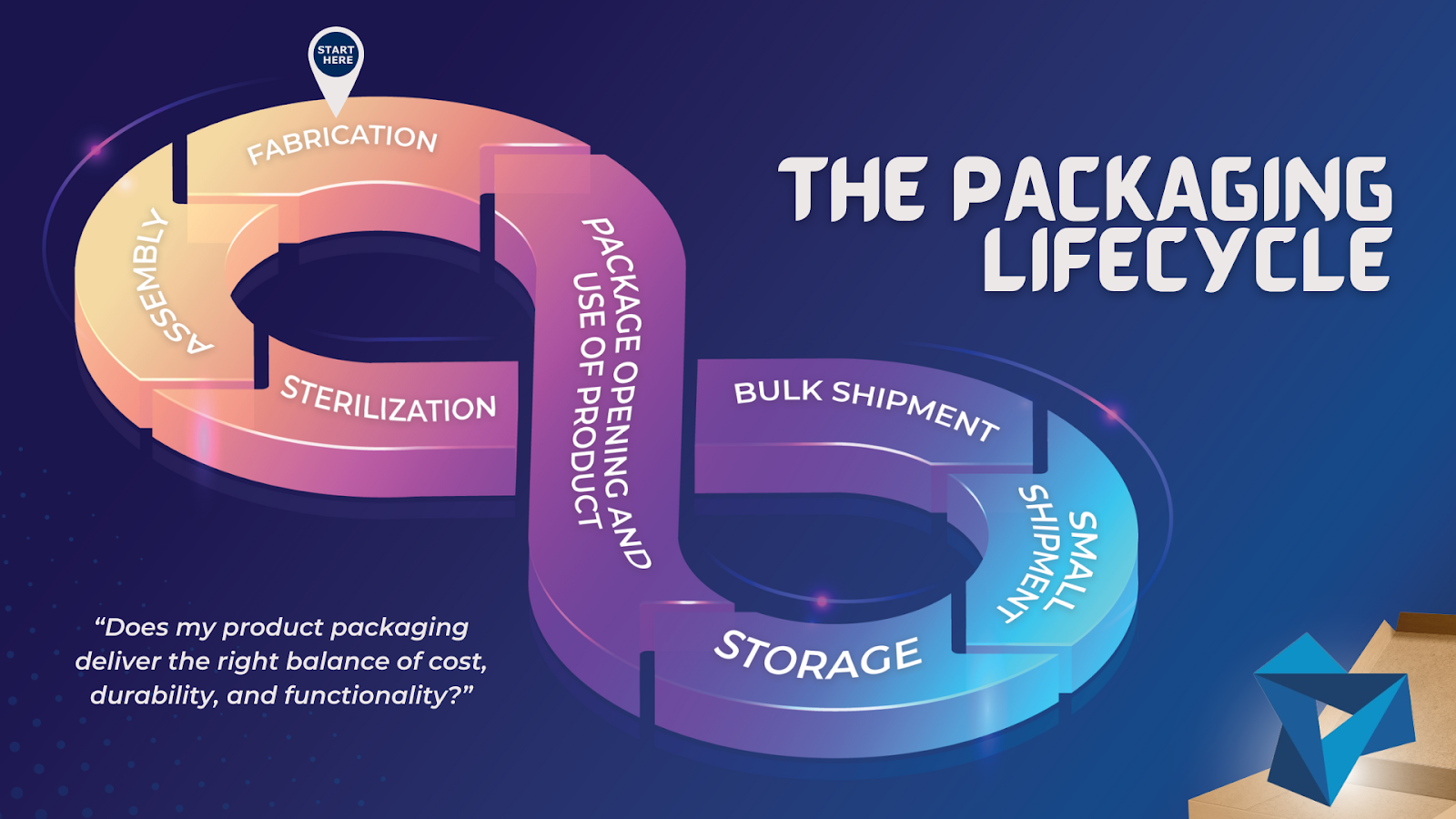
When designing a new medical, diagnostic, or life science device, the safe and effective delivery of product is just as important as its safe and effective use. Often there are several levels of packaging, one for it to be shipped in bulk, like a crate, one for it to be shipped in small quantities, like […]
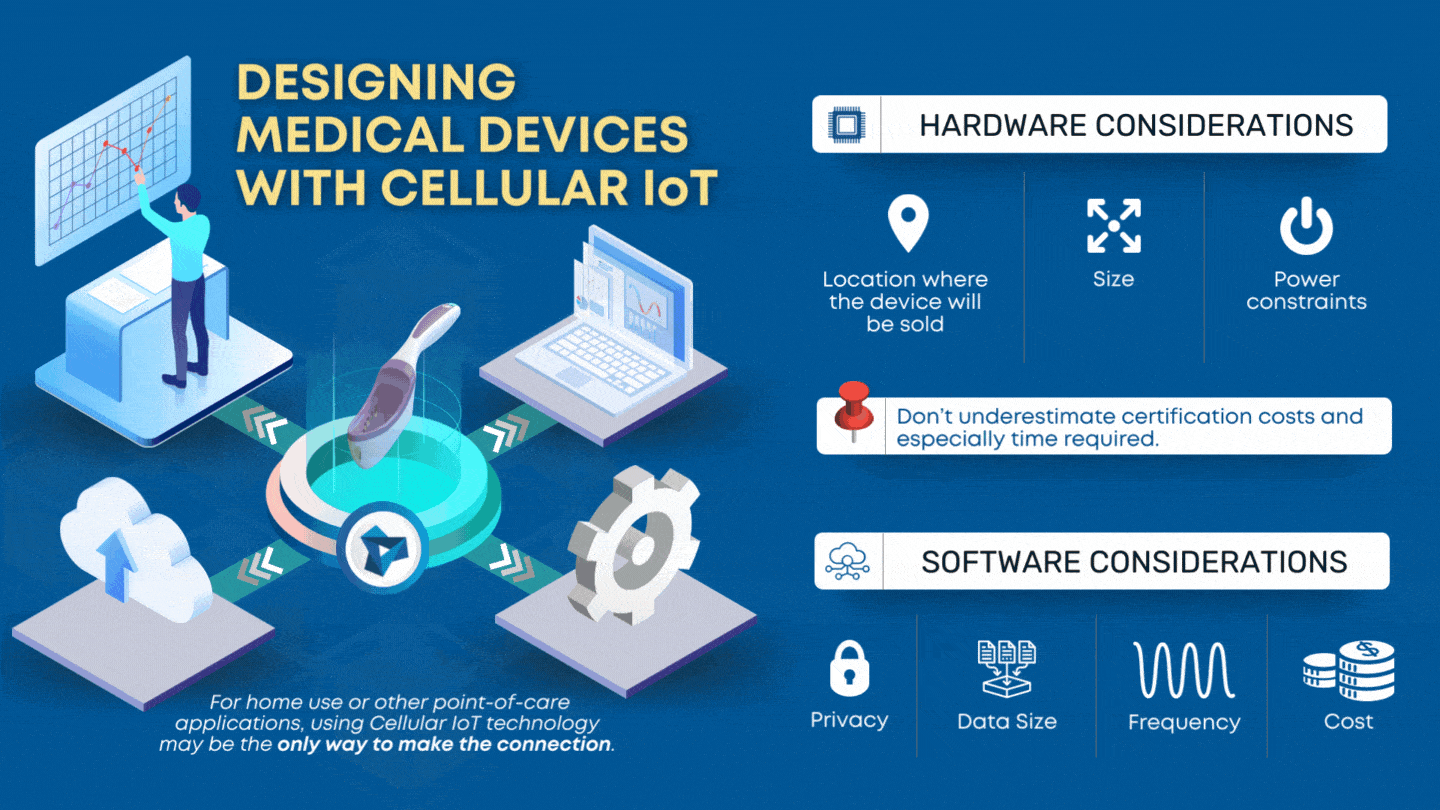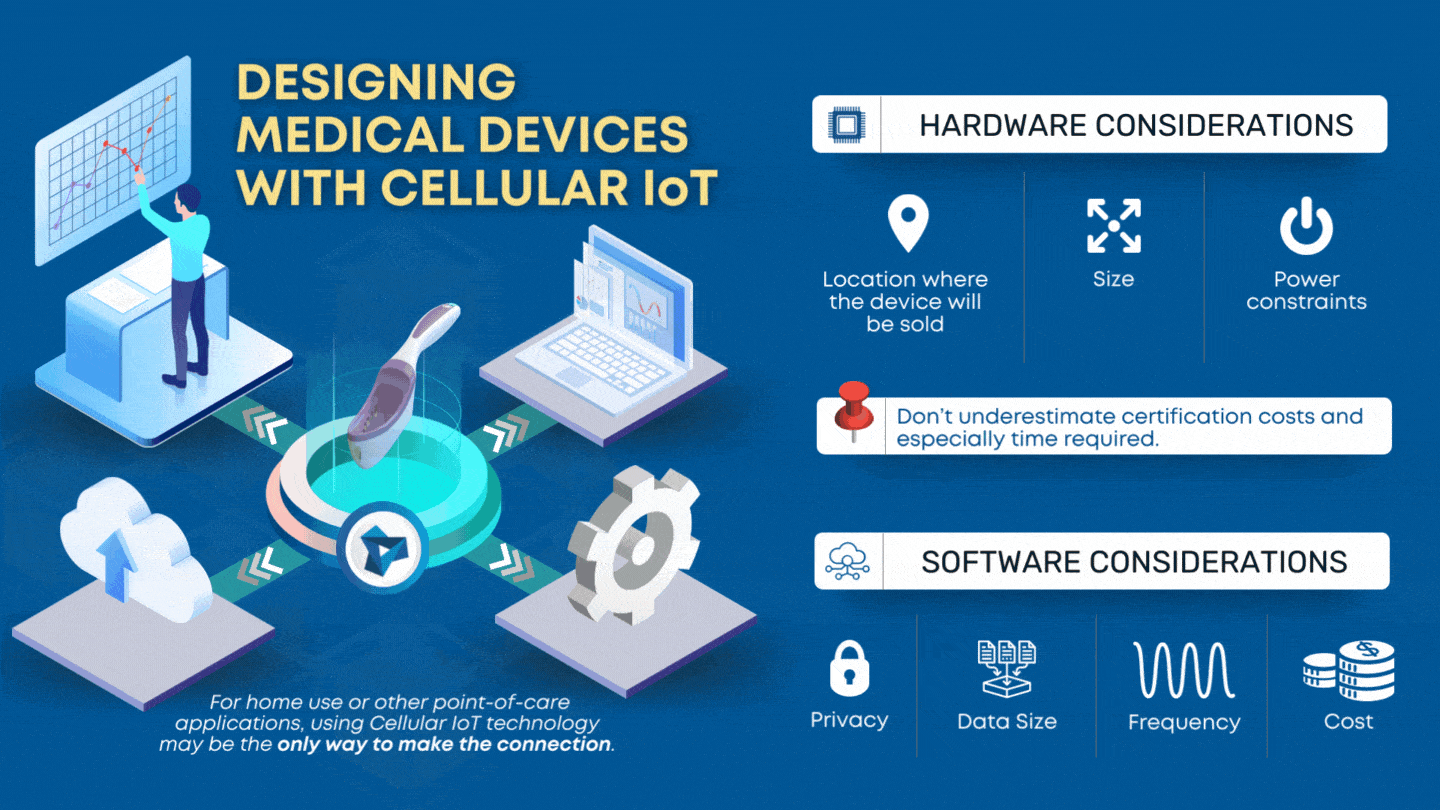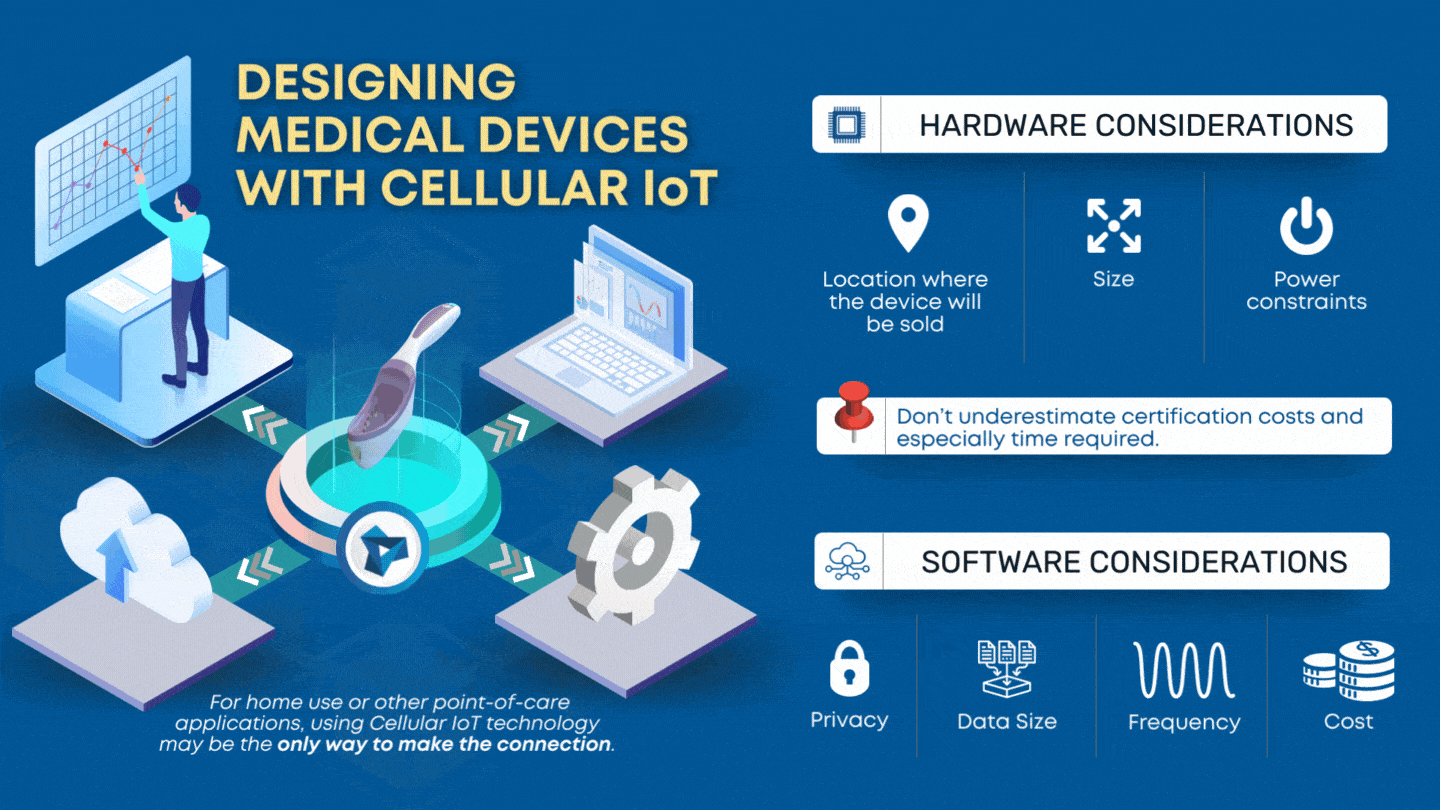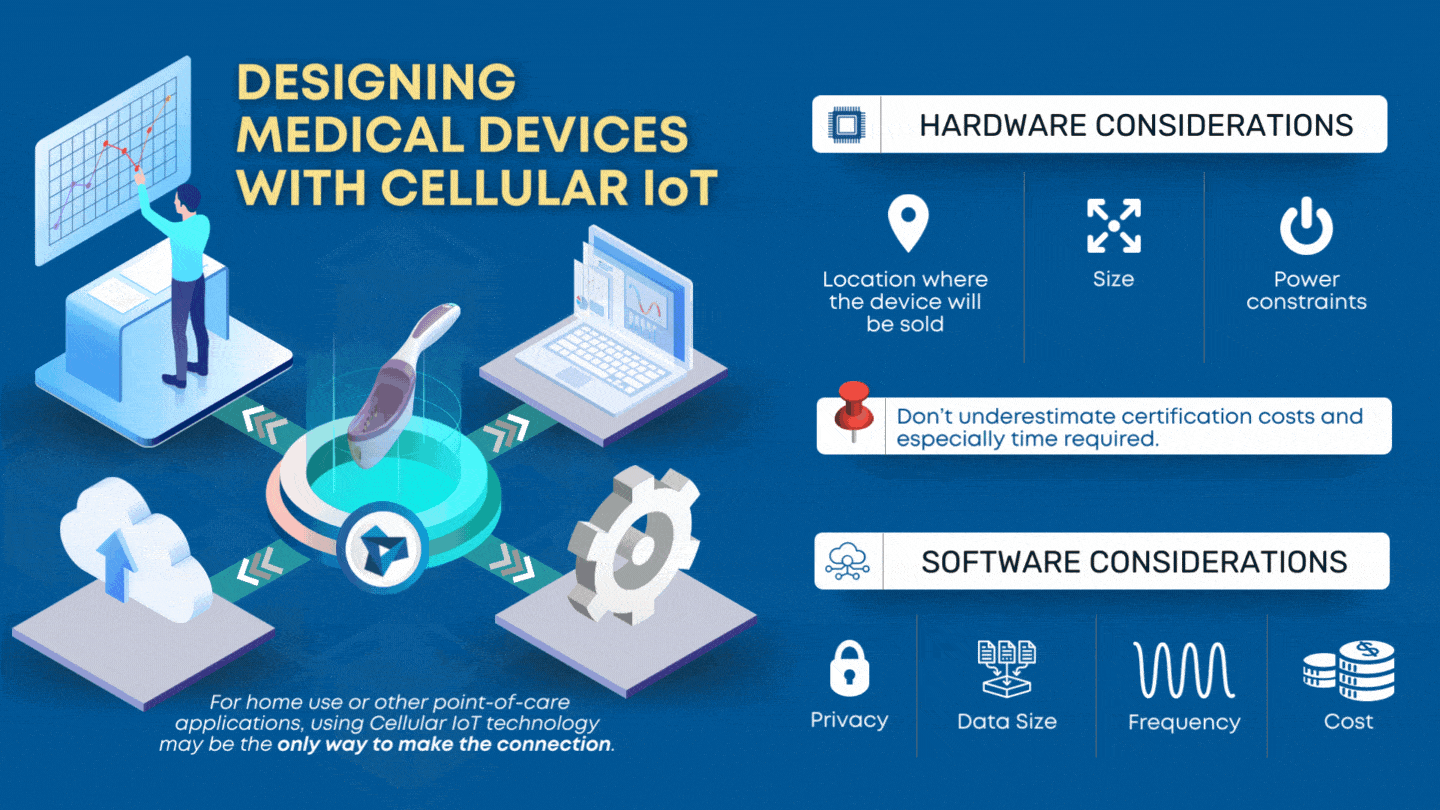
CLIA waived devices provide numerous benefits to the healthcare industry and patient care, including a broader market for manufacturers, rapid patient testing, diagnosis and treatment within an office visit, and reduced laboratory overhead and licensing costs. However, designing a CLIA waived device comes with its own challenges of ensuring the device is simple and accurate, with an insignificant […]
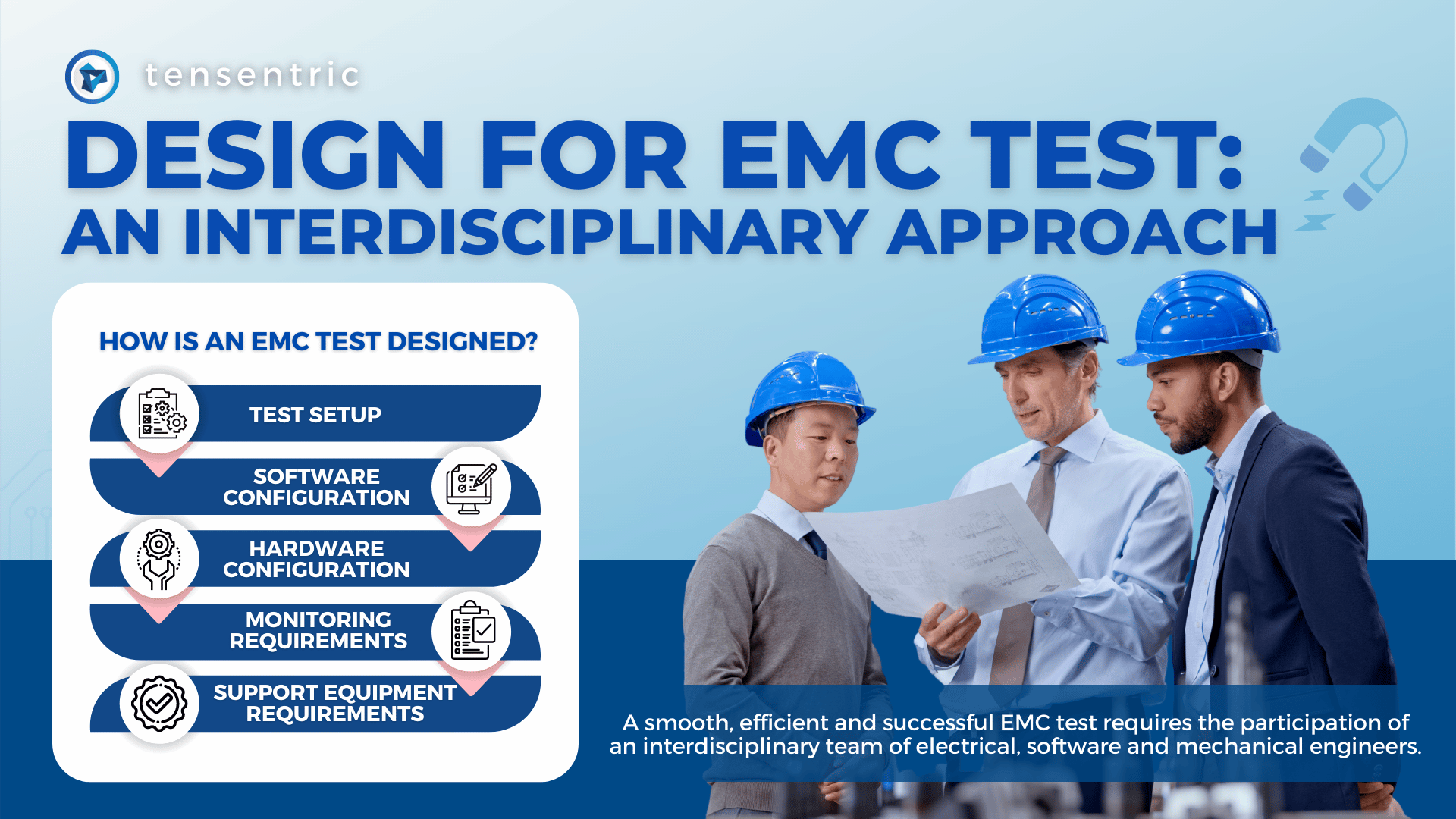
Designing a medical device to meet the IEC 60601-1-2 4th edition Electromagnetic Compatibility (EMC) standards is notoriously difficult. Making matters worse, the details of what goes on during EMC testing is cloaked in mystery to many engineers. Regardless of the difficulty, every electronic device must pass formal EMC testing before it can be sold commercially. […]